Theme: Future Prospects and Advancements of Biosimilars and Pharmaceuticals
BIOSIMILARS 2023
Conference Series will welcomes you all the participants around the globe for upcoming conference "16th International Conference on Biosimilars & Pharmaceuticals" to be held in Paris, France on June 23-24, 2023. It focuses on the theme: "Future Prospects and Advancements of Biosimilars and Pharmaceuticals". Therefore this conference provides a superb chance to debate the most recent developments within the field.
Conference Series organizes over 3000+ Global Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Health Care Professionals, Pharmacists, Druggists and Medical.
Biosimilars 2023 covers a wide range of important sessions in Biosimilars 2023 is a two-days program which includes thought inspiring Keynote Presentations, Plenary Talks, Symposiums, Special Sessions and Career Development Programs.
Biosimilars 2023 will cover the professionals gathering from Pharmaceuticals, General Surgery, Surgeons and Physicians. Also executives and eminent research personalities from the departments of Health Care Professionals, Pharmacists, Druggists. etc.shall be an integral part of this conference.
Why to attend?
With members from around the world focused on learning about global trends on Biosimilars and its advances in therapeutic and diagnostic market, this is your best opportunity to reach the largest assemblage of participants from the Biosimilars community. This particular conference conduct presentations, distributes information, conducts meetings with current and potential scientists, make a splash with new drug developments, and receive name recognition at this 2 days event. World renowned speakers, deliver speech on the most recent therapeutic and diagnostic techniques, developments in Biomarkers and Biologics. Biochemical and Biological Challenges are hallmarks of this Biosimilars 2023 conference.
Who should attend?
- Surgeons and Physicians
- General Surgery
- Oral and Maxillofacial medical procedure
- Bacteriologists
- Virologists
- Vaccine Immunologists
- Parasitologists
- Mycologists
- Pathologists
- Pharmacists
- Epidemiologists
- Health Care Professionals
- Infectious Diseases Specialists
Target Audience:
- Directors, Board Members, Presidents, Vice Presidents, Deans and Head of the Departments
- Infectious Diseases Researchers, Scientists, Faculties, Students
- Infectious Diseases Associations and Societies
- Medical Colleges
- Pharmaceutical Companies and Industries
- Medical Devices Manufacturing Companies
- Drug Manufacturing Companies and Industries
- Laboratory Technicians and Diagnostic Companies
- Business Entrepreneurs and Industrialists
- Training Institutes
- Software Developing Companies
- Data Management Companies
TRACK -1: Biosimilar and Biologics
Biosimilars are biological products that are highly similar to an already approved biologic reference product, known as the originator or reference biologic. Biosimilars are developed to be comparable in terms of safety, efficacy, and quality to the reference product. To gain approval, biosimilars undergo a comprehensive comparability exercise, which includes extensive analytical studies and clinical trials, to demonstrate that they are highly similar to the reference biologic.
Biologics are a class of medicinal products derived from living organisms or their components. They are produced using advanced biotechnology techniques and are typically large and complex molecules, such as proteins, antibodies, or nucleic acids. Biologics are used to treat various diseases, including autoimmune disorders, cancer, and chronic diseases like diabetes. Unlike traditional small molecule drugs, which are chemically synthesized, biologics are manufactured using living cells or organisms, such as bacteria or yeast.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -2: Biopharmaceuticals
Biopharmaceuticals also known as biologic drugs or biologics, are pharmaceutical products derived from biological sources, such as living cells or organisms. They are produced using biotechnology techniques and typically consist of large, complex molecules, such as proteins, peptides, antibodies, nucleic acids, or living microorganisms.
Biopharmaceuticals have revolutionized the field of medicine by offering innovative treatment options for various diseases, including cancer, autoimmune disorders, hormonal imbalances, genetic diseases, and more. They are designed to target specific molecules or pathways involved in disease processes, providing highly targeted and personalized therapies.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -3: Bio Equivalence Assessment
Bioequivalence assessment is a crucial step in the evaluation of generic drugs or biosimilar products. It compares the pharmacokinetic and sometimes pharmacodynamic properties of a generic/biosimilar drug to that of a reference drug (innovator drug) to determine if they are equivalent in terms of safety and efficacy.
The purpose of bioequivalence assessment is to demonstrate that the generic or biosimilar product is highly similar to the reference product, showing comparable bioavailability and similar therapeutic effects. It is based on the principle that if two products are bioequivalent, they can be expected to have the same therapeutic outcome when administered to patients at the same dosage
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -4: Biosimilars
Biosimilars are biological products that are highly similar to an already approved reference biologic, known as the originator or reference product. Biosimilars are developed to have a comparable quality, safety, and efficacy profile to the reference product.
Here are some key points about biosimilars:
Regulatory Approval: Biosimilars undergo a rigorous regulatory approval process to demonstrate their similarity to the reference product. The specific requirements for approval may vary across countries, but they generally involve extensive analytical and clinical studies to establish biosimilarity.
Similarity not Identical: Biosimilars are not identical copies of the reference product but are highly similar in terms of quality attributes, biological activity, and clinical performance. Minor differences may exist due to the inherent complexity of biologics and the manufacturing process, but these differences are not clinically meaningful.
Comparative Studies: To establish biosimilarity, comparative studies are conducted to evaluate the biosimilar and reference product side by side. These studies include analytical characterization, nonclinical studies, and clinical trials (including pharmacokinetic and pharmacodynamic assessments) in relevant patient populations.
Interchangeability: Some biosimilars may also seek an additional designation of "interchangeability." Interchangeable biosimilars can be substituted for the reference product without the intervention of the healthcare provider. Interchangeability status is subject to additional regulatory requirements and may not be applicable in all regions.
Cost-Effectiveness: Biosimilars offer the potential for increased access to biologic therapies and can contribute to cost savings in healthcare systems. By providing more affordable alternatives to reference products, biosimilars can enhance competition, improve patient access, and help reduce healthcare expenditures.
Therapeutic Applications: Biosimilars cover a wide range of therapeutic areas, including autoimmune diseases, oncology, diabetes, and more. They provide treatment options for patients who may have limited access to expensive biologic therapies.
Safety and Monitoring: Biosimilars undergo rigorous post-marketing surveillance to ensure ongoing safety monitoring. Robust pharmacovigilance systems are in place to track any potential adverse events or unexpected effects associated with the use of biosimilars.
It's important to note that healthcare providers play a critical role in the appropriate use of biosimilars. They need to be informed about the availability, regulatory approvals, and clinical evidence surrounding biosimilars to make informed decisions in the best interest of their patients.
Overall, biosimilars contribute to increasing patient access to biologic therapies, offering more affordable options while maintaining comparable safety and efficacy to the reference product. They provide an important avenue for expanding treatment options and promoting sustainable healthcare systems.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -5: Biologics
Biologics also known as biopharmaceuticals or biological drugs, are pharmaceutical products derived from living organisms or their components. They are produced using biotechnology techniques and can include proteins, peptides, antibodies, vaccines, gene therapies, and cellular therapies.
Here are some key points about biologics:
Source and Complexity: Biologics are derived from biological sources, such as microorganisms, animal cells, or human cells. They are often large and complex molecules, with unique three-dimensional structures and intricate manufacturing processes.
Targeted Therapies: Biologics are designed to target specific molecules, receptors, or pathways involved in disease processes. They can modulate immune responses, block signaling pathways, replace deficient proteins, or deliver therapeutic agents directly to diseased cells.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -6: Biomarkers
Biomarkers are measurable indicators or characteristics that can be objectively evaluated and used as a biological sign or measurement of normal biological processes, pathogenic processes, or responses to therapeutic interventions. They can be found in various biological sources, such as blood, urine, tissues, or imaging scans.
Here are some key points about biomarkers:
Diagnostic Biomarkers: Biomarkers can be used to diagnose diseases or conditions. They provide objective evidence of the presence or absence of a disease or indicate the likelihood of developing a disease. For example, certain proteins or genetic markers can be used as diagnostic biomarkers for cancer.
Prognostic Biomarkers: Prognostic biomarkers provide information about the likely outcome or progression of a disease. They help determine the course of the disease, predict the likelihood of recurrence, or assess the response to treatment. Prognostic biomarkers are valuable for making treatment decisions and determining patient outcomes
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK 7: Emerging Biosimilars in Therpeutics
Emerging Biosimilars in Therpeutics There are several emerging biosimilars in the field of therapeutics. While the landscape is constantly evolving, here are a few notable examples of biosimilars that have gained attention:
Adalimumab Biosimilars: Adalimumab is a widely used monoclonal antibody that targets tumor necrosis factor-alpha (TNF-α) and is used to treat autoimmune conditions such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Several biosimilar versions of adalimumab have been developed and approved in various regions.
Trastuzumab Biosimilars: Trastuzumab is a monoclonal antibody used in the treatment of HER2-positive breast cancer and gastric cancer. Biosimilars of trastuzumab have been developed and approved as cost-effective alternatives, expanding patient access to this important therapy.
Rituximab Biosimilars: Rituximab is a monoclonal antibody used to treat certain cancers, such as non-Hodgkin lymphoma, as well as autoimmune conditions like rheumatoid arthritis. Biosimilars of rituximab have been developed and approved, providing more affordable options for patients.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK -8: Clinical Development of Bio Similars
The clinical development of biosimilars involves a series of rigorous steps and studies to establish their similarity to the reference biologic and demonstrate their safety and efficacy. While the specific requirements and guidelines may vary across regulatory authorities and regions, here is a general overview of the clinical development process for biosimilars.
Analytical Comparability: Before initiating clinical trials, extensive analytical studies are conducted to compare the biosimilar and the reference biologic. These studies evaluate the physicochemical properties, biological activity, and structural characteristics of both products to ensure they are highly similar.
Nonclinical Studies: Nonclinical studies, including in vitro and in vivo preclinical studies, are conducted to further assess the similarity and potential toxicological effects of the biosimilar compared to the reference product. These studies help identify any potential differences and inform the design of clinical trials
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK-9: Pharmacovigilance Challenges in Biosimilars
Pharmacovigilance the science and activities related to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problems, presents unique challenges in the context of biosimilars. Here are some of the key challenges in pharmacovigilance for biosimilars:
Immunogenicity: Biosimilars like their reference biologics, have the potential to induce an immune response in patients. However, the immunogenicity of biosimilars may differ from that of the reference product due to minor differences in structure, impurities, or manufacturing processes. Assessing and managing immunogenicity is a critical challenge in pharmacovigilance, as it can lead to adverse events and impact patient safety and treatment efficacy.
Traceability and Product Identification: Ensuring accurate traceability and identification of specific biosimilar products can be challenging. Unlike small molecule generics, which have unique generic names and are easily traceable, biosimilars often share the same international nonproprietary name (INN) as the reference product. This can complicate the collection of accurate and specific adverse event data for individual biosimilars.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK-10: Globilization of Bio Similars
The globalization of biosimilars refers to the expansion of the development, production, regulation, and market access of biosimilar products across different regions and countries. Here are some key aspects of the globalization of biosimilars.
Regulatory Harmonization: Regulatory authorities around the world have been working to harmonize guidelines and requirements for the approval of biosimilars. This harmonization aims to establish consistent standards for demonstrating similarity, efficacy, and safety across different regions. Organizations such as the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO) play a role in promoting global harmonization efforts
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK-11:-Surgeons and Physicians
Physicians also known as doctors or medical doctors (MDs) are healthcare professionals who diagnose, treat, and manage a wide range of medical conditions. They specialize in the field of medicine and provide non-surgical medical care to patients. Physicians may choose to specialize in specific areas such as internal medicine, pediatrics, cardiology or dermatology. They work in various settings, including hospitals, clinics, private practices, and research institutions.
The responsibilities of physicians include:
- Conducting medical examinations and taking detailed patient histories to diagnose medical conditions.
- Ordering and interpreting diagnostic tests, such as blood tests, imaging studies, or biopsies.
- Developing and implementing treatment plans, which may include medications, therapies, lifestyle changes, or referrals to specialists.
- Monitoring and managing chronic conditions, providing preventive care, and promoting overall health and well-being
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK-12:- General Surgery
General surgery is a surgical specialty that focuses on the surgical treatment of a wide range of medical conditions. General surgeons are highly trained medical professionals who have expertise in performing surgical procedures on various parts of the body. They are skilled in both elective and emergency surgeries and play a crucial role in providing comprehensive patient care.
Here are some key aspects of general surgery:
- Scope: General surgery encompasses a broad range of surgical procedures involving different organs and body systems. General surgeons may perform surgeries related to the gastrointestinal tract (including the esophagus, stomach, intestines, liver, and pancreas), endocrine system (thyroid and parathyroid glands), breast, skin and soft tissues, vascular system, and more. They also handle surgical emergencies, such as appendicitis, trauma, or perforated organs.
- Surgical Procedures: General surgeons perform a variety of surgical procedures, including but not limited to:
- Abdominal surgeries: Such as appendectomy, cholecystectomy (gallbladder removal), hernia repairs, colectomy (removal of part or all of the colon), and gastric bypass.
- Breast surgeries: Including lumpectomy and mastectomy for breast cancer or other breast conditions.
- Endocrine surgeries: Such as thyroidectomy (removal of the thyroid gland) or parathyroidectomy (removal of parathyroid glands).
- Skin and soft tissue surgeries: Including removal of skin lesions, hernia repairs, or treatment of abscesses.
- Vascular surgeries: Such as the placement of vascular access for dialysis or the treatment of varicose veins
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
TRACK-13:- Oral and Maxillofacial medical procedure
Oral and Maxillofacial Surgery (OMS) is a surgical specialty that focuses on the diagnosis and treatment of conditions related to the mouth, jaws, face, and associated structures. It is a combination of dentistry, medicine, and surgery. Oral and Maxillofacial surgeons are highly trained professionals who specialize in performing surgical procedures in this field. Here are some key aspects of Oral and Maxillofacial Surgery
- Scope: Oral and Maxillofacial surgeons are involved in the diagnosis and treatment of a wide range of conditions, including:
- Dental extractions, including impacted wisdom teeth.
- Dental implant placement and bone grafting procedures.
- Corrective jaw surgery (orthognathic surgery) to correct jaw deformities or misalignments.
- Treatment of facial trauma, such as fractures of the jaw, cheekbones, or nose.
- Removal of benign or malignant tumors in the oral and facial region.
- Treatment of temporomandibular joint (TMJ) disorders and facial pain.
- Management of congenital facial deformities, such as cleft lip and palate.
- Facial cosmetic surgeries, such as facial rejuvenation procedures or chin augmentation.
Biosimilars Conferences | Pharmaceutical and Biopharmaceutical Research | Biologics and Biomarkers Conferences | Paris Conferences | Paris Biosimilars Conferences
After the successful completion of the Biosimilars 2020 & 2021, We are pleased to welcome you to the "16thInternational Conference on Biosimilars And Pharmaceuticals" The conference is scheduled to take place during June 23-24, 2023 in the beautiful city of Paris, France. This Biosimilars 2023 Conference will give you exemplary experience and great insights in the field of research.
As per market researchers the global Biosimilars Market by Product (Recombinant Non-Glycosylated Proteins (Insulin, HGH, Interferon), Glycosylated (MAB, EPO), Peptides (Glucagon, Calcitonin)), Manufacturing Type (In-house, Contract), Disease (Oncology, Autoimmune) - Global Forecast to 2023", The biosimilars market is expected to reach USD 23.63 Billion by 2023 from USD 5.95 Billion in 2018, at a CAGR of 31.7%. Granulocyte colony-stimulating factors (G-CSFs) reached $379.3 million in 2013. This segment is expected to increase from $453.6 million in 2014 to $1.1 billion by 2021, a CAGR of 20.2% from 2014 to 2021.
Importance & Scope:-
The European-based pharmaceutical industry makes a major contribution to the Europe, not just in financial terms but also in terms of high-trait employment. Globally Pharma Market ranges from $870-$900 billion and in Europe $260-$280 billion.
A global biosimilars strategy:-
Developed markets: Developed markets, with the exception of the United States, represent the greatest biosimilars presence today. Most biosimilars manufacturers have been and remain focused on the developed markets – whether it is for their historic and current opportunities (EU) or for their future market potential (United States, Japan). Dedicated regulatory pathways set the foundation for stringent, abbreviated approval processes which, in turn, have fed investor enthusiasm. Biosimilars adoption in developed markets has been primarily payer-driven, especially in European markets, given payers’ urgent, unmet need to contain public health care expenditures. Further market uptake has been slowed by prescribers’ skepticism and low patient awareness. Still, developed markets continue to have the highest number of biosimilars molecules in development – estimated at 29 in Europe, 19 in the United States and 7 in Japan.
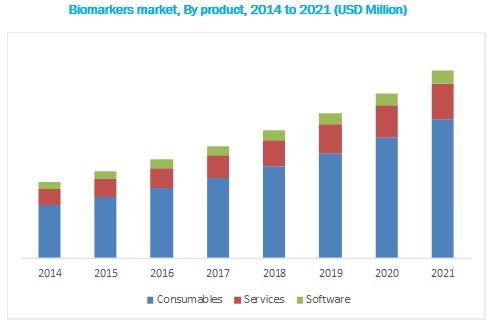
Based on product, the biomarkers market is segmented into consumables, services, and software. The consumables segment accounted for the largest share of the global biomarkers market in 2016, primarily due to the increasing use of reagent kits and assays for biomarker testing and the higher frequency of purchase of consumables. Although the price of consumables is lower than that of screening and automation instruments, consumables have a higher share in this market owing to their larger sales volume. As such, growth in the overall biomarkers market will be the most important driver for this product segment. Furthermore, the introduction of singleplex platforms, multiplex platforms, and automated ELISA systems that require assay kits is expected to aid the growth of this product segment.
14th International Conference on Biosimilars and Pharma Innovations was organized during October 12-13, 2020 in Prague, Czech Republic with the support and contribution of the Organizing Committee Members. Firstly we must thank you for trusting us and participating at EuroBiosimilars2020, a global platform to discuss various important aspects of Biosimilars & Pharmaceuticals.
The conference proceedings were carried out through various Scientific-sessions and plenary lectures, of which the following Speakers were highlighted as Keynote speakers: Ivo Abraham University of Arizona (USA), Steven Lehrer Optimal Strategix Group (USA), Russell Reeve Quintiles Inc. (USA). Scientific sessions discussed during webinar are about Biosimilars and Biologics, Biologics, Biomarkers, Globalization Of BioSimilars
13th International Conference Of Biosimilars and Biologics during March 18-19, 2019 in Amsterdam ,Netherlands. It was a great success, where eminent speakers from various reputed institutions and organizations with their resplendent presence addressed the gathering. The adepts who promulgated the theme with their exquisite talks were: Russell Reeve(USA) ,Arturo Solis Herrera(Mexico), Farrokh Janabi-Sharifi (Canada), Lin Zhou (China). Scientific sessions discussed during conference are about Biosimilars and Biologics, Biologics, Biomarkers, Globalization Of BioSimilars
12th Asian Biologics and Biosimilar Congress during August 20-21, 2018 in Tokyo, Japan. It was a great success, where eminent speakers from various reputed institutions and organizations with their resplendent presence addressed the gathering. The adepts who promulgated the theme with their exquisite talks were: James Gunderso (USA), Peter Kalinka (USA), Sarfaraz Niazi (USA), Rodica Olteanu (Romania). Scientific sessions discussed during conference are about Biosimilars and Biologics, Biologics, Biomarkers, Globalization Of BioSimilars
10th International Conference And Exhibition On Biologics and Biosimilars during October 16-17, 2017 in San Francisco, USA. It was a great success, where eminent speakers from various reputed institutions and organizations with their resplendent presence addressed the gathering. The adepts who promulgated the theme with their exquisite talks were Yashwant Pathak (USA), Fiona M Greer (Switherland), Pawan Saharan (India). Scientific sessions discussed during conference are about Biosimilars and Biologics, Biologics, Biomarkers, Globalization Of BioSimilars
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | June 23-24, 2023 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Molecular Biomarkers & Diagnosis
- Journal of Biomedical and Pharmaceutical Sciences
- Biomarkers Journal
Abstracts will be provided with Digital Object Identifier by
























